DIO News
DIO, launched a new Product: Short Implant
Korea 27 Oct, 2020
- Short-length implants overcome severe alveolar bone resorption cases
-
On the 2nd of October, DIO (CEO Jinbaek Kim), a company leading the digital dentistry, launched a new UF(Ⅱ) Short Implant (hereinafter referred to as Short Implant) optimized for patients with severe alveolar bone resorption.
In recent years, when a teeth becomes damaged, implants are used more instead of the traditional fixed prosthesis. The most important thing at this time is the height and quality of the available bone, and they greatly affect the initial stability during implant placement. Therefore, when placing an implant in a patient with insufficient available bone, additional surgery to enhance the height of the bone is often required.
However, in this case, placing a short implant without additional surgery can also be a method. Since this procedure can be completed relatively simply, this is an advantage that both the patient and the operator can relieve the burden of.
DIO paid attention to this point and has continued to study short implants. By improving the design and surface, DIO was able to complete the development of a short implant that allows implant placement even when bone resorption is severe.
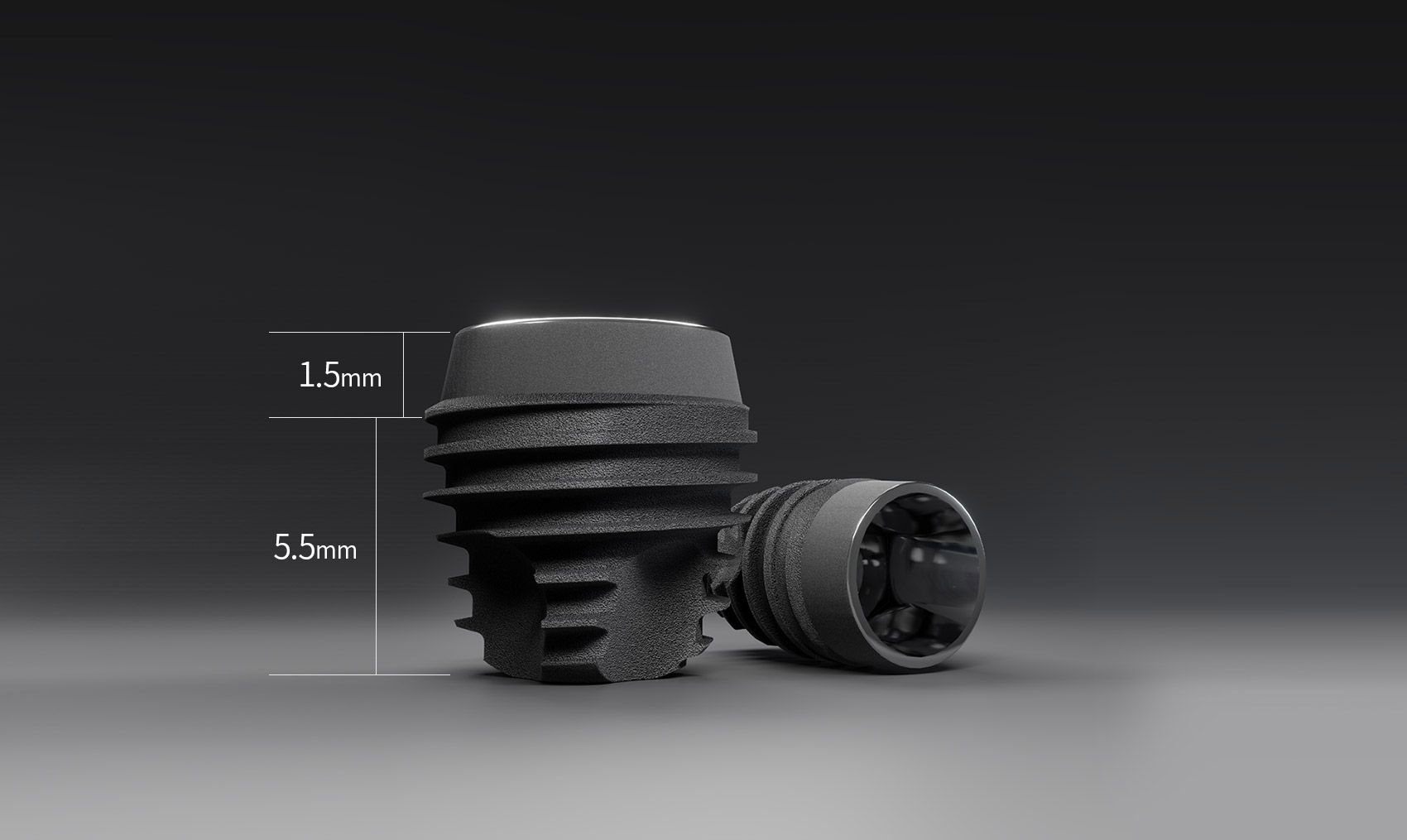
Short Implant is an implant with a thread length of 5.5mm.
Previously, the shortest length was 7mm, but this was further improved with the development of a shorter length implant. Short drills for short implant placement have been added to the current UF(Ⅱ) Surgical KIT and Master KIT, and a surgical guide was added for the DIOnavi. users. It has been updated to enable more precise Short Implant placement using Master KIT and Wide KIT.
Short implant has the advantage of having a very satisfying feeling of placement and being able to easily secure initial stability. Also, because the ‘Bevel’ part can be exposed to the gums, the convenience of the surgeon when placing an implant has increased.
In particular, the success rate can be increased in cases where it is difficult to perform surgery with an implant of a general size, such as when the alveolar bone resorption is severe, so it is expected that the use and interest of the surgeons will increase.
Short Implant has already completed all clinical tests. As a result of clinical tests for clinicians with extensive clinical experience, it was confirmed that initial fixation was well achieved despite the case of severe alveolar bone resorption and poor bone quality.
In addition, the self-tapping ability was also excellent, indicating that the satisfaction level was very high.
Mr.Sang-Oh Park, Director of DIO Implant Development Team said, "We will focus all our capabilities to continuously provide more convenient and safe products and solutions to medical staff even in various clinical environments and in difficult cases."